

Views: 0 Author: Site Editor Publish Time: 2025-10-30 Origin: Site









Barium sulfate (BaSO₄) is a chemical compound with wide-ranging applications across chemistry, industry, and medicine. Known for its bright white color, high density, and chemical stability, BaSO₄ is used as a filler in plastics, pigments in paints, and even as a contrast agent in medical imaging. While it is commercially available in solid form, one of the most fundamental aspects of barium sulfate in chemistry is its formation as a precipitate. Understanding this process is critical for chemists, industrial operators, and laboratory technicians alike.
In chemical reactions, a precipitate refers to a solid that forms when two soluble compounds react in solution. The formation of BaSO₄ as a precipitate has important implications, from determining sulfate concentrations in analytical chemistry to producing pure materials for industrial and medical use. Knowing whether barium sulfate forms a precipitate helps researchers understand reaction mechanisms, ensure product quality, and maintain safety standards when handling barium compounds.
A precipitate is a solid substance that emerges from a solution during a chemical reaction, often as the result of combining two soluble compounds that produce an insoluble product. Unlike dissolved ions that move freely in solution, precipitates aggregate to form solid particles that can settle out or be separated by filtration or centrifugation.
Precipitation reactions are essential in analytical chemistry, industrial manufacturing, and research. Common examples include the reaction of silver nitrate with sodium chloride to form silver chloride (AgCl) and the reaction of calcium chloride with sodium carbonate to form calcium carbonate (CaCO₃). These reactions are characterized by the sudden appearance of a solid within a previously clear solution.
The ability of a compound to precipitate is often predicted using its solubility product constant (Ksp). The Ksp defines the maximum concentration of ions that can exist in solution without forming a solid. If the ionic product exceeds this value, the solution becomes supersaturated, and the compound precipitates. For barium sulfate, the solubility product is extremely low, meaning even small concentrations of barium and sulfate ions in solution can lead to precipitation.
Barium sulfate is most commonly formed through a reaction between a soluble barium salt and a sulfate salt in aqueous solution. For example:
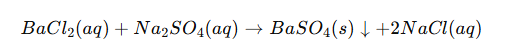
Similarly, barium nitrate can react with sulfuric acid or sodium sulfate to produce the same precipitate:
![]()
The resulting barium sulfate is a fine, white, dense solid that is highly insoluble in water. This reaction demonstrates the classic behavior of precipitation, as the product separates immediately from the solution due to its extremely low solubility.
The physical characteristics of the precipitate include a bright white color, high density, and fine particle size. Depending on reaction conditions and the method of preparation, BaSO₄ may appear as microcrystalline aggregates or a colloidal suspension.
High concentration of reactants
Low solubility environments
Ambient or slightly elevated temperatures
Absence of interfering ions or complexing agents
Several factors affect the formation, size, and quality of barium sulfate precipitate:
The ratio of barium ions to sulfate ions influences how completely the precipitation reaction occurs. A slight excess of either ion can affect the particle size and homogeneity of the precipitate. In industrial or lab-scale reactions, carefully controlled stoichiometry ensures consistent and reproducible results.
Although BaSO₄ is highly insoluble, temperature and the ionic composition of the solution can subtly influence the rate of nucleation and crystal growth. Higher temperatures may accelerate the precipitation process, while high ionic strength can impact the size and aggregation of particles.
Impurities in the reactants, or the presence of additives, can inhibit crystal growth, change particle morphology, or affect sedimentation. In some industrial applications, surface modifiers are added to control particle size, prevent agglomeration, and enhance dispersibility in plastics, coatings, or medical suspensions.

In a laboratory setting, barium sulfate precipitation can be observed and confirmed using multiple techniques:
BaSO₄ appears as a bright white solid immediately upon formation. In well-mixed solutions, the precipitate may appear colloidal and suspended, eventually settling due to gravity.
To isolate the precipitate, chemists use filtration or centrifugation. Filtration captures the solid on a medium, such as filter paper or membrane, while centrifugation accelerates the settling of fine particles for easier separation.
Gravimetric analysis is a common method to confirm BaSO₄ precipitation. In this technique, the precipitate is filtered, dried, and weighed to determine the amount of sulfate or barium in the original solution. Advanced methods like X-ray diffraction (XRD) or electron microscopy can reveal the crystalline structure and particle morphology, distinguishing microcrystalline from colloidal forms.
Barium sulfate precipitation plays a crucial role in quantitative laboratory analysis. By forming a pure, stable precipitate, chemists can accurately determine sulfate concentrations in water, soil, industrial effluents, or biological samples. The low solubility of BaSO₄ ensures high precision in gravimetric and volumetric assays.
In industry, barium sulfate precipitates are utilized in several ways:
Water Treatment: BaSO₄ precipitation helps remove sulfate ions from wastewater, preventing scale formation and environmental contamination.
Pigment Production: Precipitated BaSO₄ is used as a filler and pigment in paints, coatings, and plastics due to its whiteness, chemical inertness, and high density.
Polymer Fillers: BaSO₄ improves hardness, surface gloss, and dimensional stability in plastics and rubber products.
Although pharmaceutical-grade BaSO₄ used in X-ray imaging is produced under strict standards, the concept of precipitation is relevant. Laboratory precipitation ensures high purity and controlled particle size, critical for creating suspensions that coat the gastrointestinal tract safely and provide clear radiographic images. The precipitated form’s fine, uniform particles help prevent clumping and ensure even distribution in contrast media.
It is important to clarify several common misconceptions regarding BaSO₄:
Not Always Added as Solid: While BaSO₄ is available as a solid, in many reactions it is generated in situ via precipitation. This approach ensures higher purity and precise control over particle characteristics.
Comparison with Other Barium Compounds: Soluble barium salts, like barium chloride or nitrate, do not precipitate on their own but form BaSO₄ when combined with sulfate ions. This distinguishes them in terms of chemical handling and applications.
Purity and Consistency: Precipitation ensures that BaSO₄ is free from soluble contaminants, making it ideal for analytical, industrial, and medical purposes.
Understanding the precipitation of barium sulfate offers numerous benefits:
Improved Experimental Accuracy: Predictable formation of pure BaSO₄ allows for precise gravimetric and analytical measurements.
Optimized Industrial Performance: Controlled precipitation ensures uniform particle size, critical for fillers, coatings, and plastics.
Safer Handling: Precipitated BaSO₄ is non-soluble and non-toxic, reducing the risk associated with soluble barium compounds.
Consistency in Medical Applications: Uniform particle size in X-ray contrast agents ensures reliable imaging results.
Q1: What does it mean when BaSO₄ precipitates?
It means that barium ions and sulfate ions in solution have combined to form a solid, insoluble compound that separates from the liquid.
Q2: Can all barium salts form a precipitate with sulfate ions?
Most soluble barium salts, such as barium chloride and barium nitrate, will form a BaSO₄ precipitate when combined with sulfate salts.
Q3: How fast does barium sulfate precipitate in solution?
Precipitation occurs almost immediately upon mixing solutions containing sufficient concentrations of barium and sulfate ions.
Q4: Can the precipitate be used directly in industrial applications?
Yes, precipitated BaSO₄ can be processed and used as a filler, pigment, or in other applications, depending on particle size and purity.
Q5: How is precipitated BaSO₄ different from natural barite?
Natural barite is mined and often requires purification. Precipitated BaSO₄ is generated in controlled conditions, resulting in higher purity and uniform particle size.
Barium sulfate is a classic example of a precipitate, formed when soluble barium salts react with sulfate ions. Its insolubility, chemical stability, and fine particle characteristics make it essential across laboratory analyses, industrial manufacturing, and medical applications, including X-ray imaging. Understanding the precipitation process ensures that the resulting BaSO₄ is of high purity, exhibits consistent performance, and can be handled safely in various settings.
For professionals and industries seeking reliable, high-quality barium sulfate, partnering with a reputable supplier is critical. Qingdao Red Butterfly Precision Materials Co., Ltd. offers carefully processed BaSO₄ with controlled particle size and superior chemical purity, making it suitable for analytical, industrial, and medical purposes. By sourcing from a trusted supplier, users can achieve optimal results in experiments, manufacturing processes, and diagnostic applications, while minimizing the risk of contamination or inconsistent performance. Contacting the company directly provides access to expert guidance, customized grades, and reliable product support for any barium sulfate requirement.
